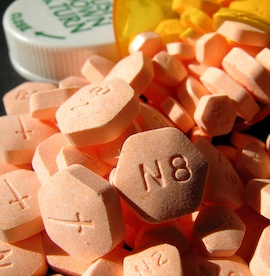
An implantable version of withdrawal treatment drug buprenorphine (also known as Suboxone) was approved on Thursday by an advisory committee to the Food and Drug Administration. Developed by Titan Pharmaceuticals under the brand name Probuphine, the implantable rods administer a steady dose of buprenorphine to patients withdrawing from opiates, such as heroin or prescription painkillers. Each rod is about the size of a matchstick, and is implanted under the upper arm for up to six months. “Opioid dependence is a rapidly growing public health concern and we remain committed to expanding the treatment options available to physicians and patients to manage this chronic condition,” said Titan in a statement. A final decision on Probuphine is expected to be reached by April 30th. Though many medical and treatment experts support the implant, there are some concerns that the product is immature and the dosage needs to be tweaked. “We need a dose-ranging study,” said panelist Laura McNicholas, MD, PhD, of the University of Pennsylvania, who voted against approval. “I am a supporter of the product and I think it’s approvable, but I think this is premature.” Overall, the panelists agreed that there is hope for the drug, and that it could be useful for those suffering from opioid addiction. “It seems that this is a product with great utility, although there are deficiencies in terms of dose-finding and the REMS [risk evaluation and mitigation strategy],” said committee chairperson David Brent, MD, of the University of Pittsburgh. “But it seems the need for a product such as this on the market exceeds those deficiencies.”
